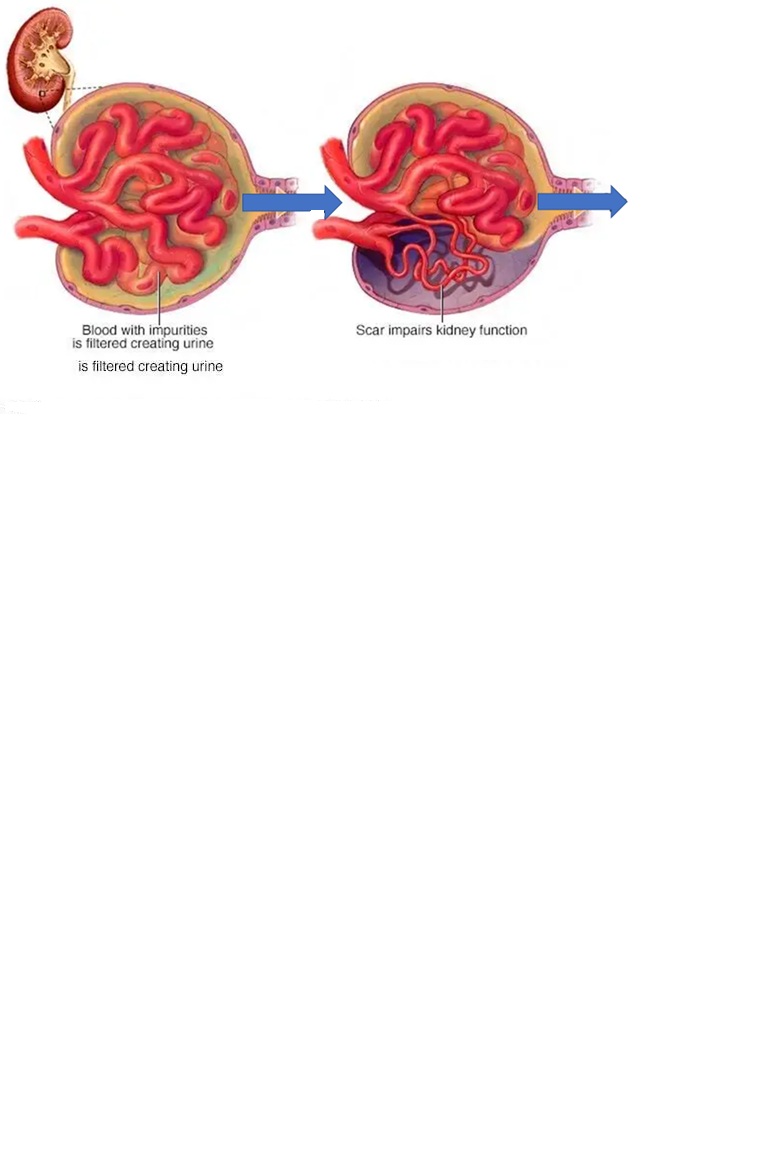
President’s
Message
Dear colleagues and friends, welcome to the Arab Society of Nephrology & Renal Transplantation. Together, we strive to elevate kidney care, advance research, and empower every patient across our region. I invite you to explore, connect, and contribute to this vibrant community.

About ASNRT
Founded in 1985, the Arab Society of Nephrology & Renal Transplantation (ASNRT) is a non-profit scientific body dedicated to advancing kidney care, research, and education across the Arab world. We connect professionals, empower patients, and shape the future of renal health in the region.
Representing a unified Arab nephrology network across 3 continents.
Building trust and advancing renal care since 1985.
A vibrant multidpnary commnity of nephrts, surgeons & nurses.
Fostering education, collaboration, and regional innovation.